Bispecific Antibody Clinical Trial Pipeline Gains Momentum: 180+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
The bispecific antibody market is experiencing rapid growth, primarily driven by the increasing prevalence of complex chronic diseases, such as cancer and autoimmune disorders, for which conventional therapies often fall short. These innovative molecules offer dual-targeting capabilities, enabling superior efficacy and therapeutic precision. Ongoing R&D advancements and increasing regulatory approvals are driving the clinical adoption of these technologies.
New York, USA, July 07, 2025 (GLOBE NEWSWIRE) -- Bispecific Antibody Clinical Trial Pipeline Gains Momentum: 180+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
The bispecific antibody market is experiencing rapid growth, primarily driven by the increasing prevalence of complex chronic diseases, such as cancer and autoimmune disorders, for which conventional therapies often fall short. These innovative molecules offer dual-targeting capabilities, enabling superior efficacy and therapeutic precision. Ongoing R&D advancements and increasing regulatory approvals are driving the clinical adoption of these technologies.
DelveInsight’s 'Bispecific Antibody Competitive Landscape – 2025' report provides comprehensive global coverage of available, marketed, and pipeline bispecific antibodies in various stages of clinical development, major pharmaceutical companies working to advance the pipeline space and future growth potential of the bispecific antibody competitive domain.
Key Takeaways from the Bispecific Antibody Pipeline Report
- Over 180+ companies are evaluating 250+ bispecific antibodies in various stages of development, and their anticipated acceptance in the bispecific antibody market would significantly increase market revenue.
- Leading bispecific antibody companies such as Instil Bio, Akeso Biopharma, Janssen, Amgen, Akeso, Zymeworks, Roche, IGM Biosciences, MacroGenics, Provention Bio, Jiangsu Alphamab Biopharmaceuticals, Sichuan Baili Pharmaceutical, Regeneron Pharmaceuticals, Boehringer Ingelheim, ABL Bio, Zymeworks, Compass Therapeutics, EpimAb Biotherapeutics, Betta Pharmaceuticals, Affimed Therapeutics, Novo Nordisk, and others are evaluating novel bispecific antibodies to improve the treatment landscape.
- Key bispecific antibodies in the pipeline in various stages of development include AXN-2510, AK-104, Amivantamab, Blinatumomab, Ivonescimab, Zanidatamab, Glofitamab, Imvotamab, MGD024, PRV 3279, KN-046, SI-B001, REGN-5458, BI-905711, ABL 102, ZW171, CTX-8371, EMB-01, MCLA-129, AFM13, Mim8, and others.
Request a sample and discover the recent advances in the bispecific antibody market @ Bispecific Antibody Competitive Landscape Report
Bispecific Antibody Overview
Bispecific antibodies (bsAbs) are engineered molecules that simultaneously bind two different antigens. One binding site typically targets the CD3 receptor on cytotoxic T lymphocytes, thereby activating them, while the other site is directed toward tumor-associated antigens such as CD19, CD20, CD33, CD123, HER2, EpCAM, BCMA, CEA, and others. This dual targeting brings T cells into close proximity with tumor cells, leading to T cell activation and tumor cell destruction.
Beyond cancer treatment, bispecific antibodies have been developed for various other diseases. For instance, in osteoporosis, a bispecific antibody targets and inhibits sclerostin and Dkk1, both negative regulators of the Wnt signaling pathway, thereby promoting osteoblast activity and bone growth. Another example is ACE910 (emicizumab), which bridges coagulation factors IX and X to restore hemostasis in patients with hemophilia A by enhancing the coagulation cascade. A different bispecific antibody under investigation for Alzheimer’s disease targets both the transferrin receptor (facilitating blood–brain barrier transport) and BACE1, an enzyme involved in amyloid-beta production.
Bispecific antibodies often target cytokines such as TNF, IL1, IL4, IL14, IL17, IL23, and others in autoimmune diseases. Using two separate monoclonal antibodies (mAbs) against cytokines has been associated with increased side effects without added efficacy. Bispecific antibodies, however, can combine two anti-cytokine specificities in a single molecule, potentially offering greater therapeutic benefits. For example, IL17, IL23, IL6, and TNF are key targets in psoriasis. ABT122, which targets both TNFα and IL17A, has shown clinical benefit in rheumatoid and psoriatic arthritis. In contrast, COVA322, which has the same targets, was discontinued in early clinical trials due to safety issues. ABT981, targeting IL1α and IL1β, is aimed at treating osteoarthritis by neutralizing inflammatory cytokines found in joint tissues.
Bispecific antibodies offer notable advantages over traditional monospecific antibodies. They can more effectively guide immune effector cells to tumors, increasing cytotoxic responses. Their ability to bind two distinct antigens provides greater target specificity and may enhance therapeutic efficacy. Compared to using a combination of two separate monoclonal antibodies, bispecifics can reduce development and clinical costs. Moreover, since some disease-driving molecules act through multiple pathways and many tumors express multiple growth-promoting receptors, dual targeting on the same cell may boost antiproliferative effects and help prevent resistance.
Approved Bispecific Antibody Drugs Profile
Amivantamab: Janssen
Amivantamab is a fully human bispecific antibody that targets two well-established cancer-related proteins, EGFR and Met. In July 2012, Genmab partnered with Janssen Biotech, Inc. to develop bispecific antibodies utilizing Genmab’s DuoBody technology platform. Both antibody libraries used in the creation of amivantamab were developed by Genmab, and the final antibody combination was selected jointly by Genmab and Janssen. Janssen subsequently led the development process. In 2021, the U.S. FDA approved amivantamab-vmjw (marketed as RYBREVANT) for treating adults with locally advanced or metastatic non-small cell lung cancer harboring EGFR Exon 20 insertion mutations, following progression after platinum-based chemotherapy. This marked the first regulatory approval for a therapeutic developed using Genmab’s proprietary DuoBody bispecific technology.
Blinatumomab: Amgen
Blinatumomab (AMG 103) is a bispecific T cell engager (BiTE®) antibody that harnesses the body’s T cells to target and eliminate cells expressing CD19, a protein commonly found on B-cell derived leukemias and lymphomas. This engineered antibody binds to two distinct targets at once, bringing T cells into close proximity with cancer cells to promote their destruction. As the first BiTE antibody developed, Blinatumomab has received orphan drug status from the U.S. FDA for treating acute lymphoblastic leukemia, chronic lymphocytic leukemia, hairy cell leukemia, prolymphocytic leukemia, and indolent B-cell lymphoma. The European Medicines Agency has granted similar designation for ALL, CLL, mantle cell leukemia, and indolent B-cell lymphoma. It is approved for use in treating Precursor B-cell lymphoblastic leukemia-lymphoma and is also under investigation for indications including Non-Hodgkin’s lymphoma and diffuse large B-cell lymphoma.
Find out more about the FDA-approved bispecific antibody list @ Bispecific Antibody Drugs
Bispecific Antibody Market Dynamics
The bispecific antibody market is experiencing dynamic growth, driven by advances in antibody engineering and increasing demand for more effective, targeted cancer therapies. Unlike traditional monoclonal antibodies, bispecific antibodies are designed to simultaneously bind two different antigens or epitopes, enabling novel mechanisms of action such as T-cell redirection, dual signaling pathway inhibition, or tumor microenvironment modulation. This unique functionality is particularly valuable in oncology, where BsAbs can bridge T-cells with tumor cells, leading to more potent and specific immune responses.
The market dynamics are further shaped by a surge in R&D investment and a growing pipeline of bispecific candidates. Leading companies, including Roche, Regeneron, Genmab, and Zymeworks, among others, are actively developing platforms that improve BsAb stability, manufacturability, and half-life. Emerging formats such as knob-into-hole, CrossMab, and DuoBody are addressing previous limitations related to molecule size, immunogenicity, and production complexity, thereby accelerating the pace of development and regulatory approval.
Despite the scientific excitement, the bispecific antibody market faces several challenges. Manufacturing remains technically complex and cost-intensive, particularly when producing asymmetric or multivalent molecules. Additionally, safety concerns such as cytokine release syndrome (CRS), off-target effects, and immunogenicity continue to be significant barriers. As more bispecifics enter clinical trials, patient stratification and biomarker development are becoming increasingly important to ensure therapeutic efficacy and minimize adverse events.
In summary, the bispecific antibody market is at a critical inflection point, with tremendous potential to transform therapeutic strategies across multiple disease areas. Scientific innovation, regulatory support, and strategic investment are aligning to drive both clinical and commercial success. However, the path forward will require continued advances in platform technology, manufacturing optimization, and risk mitigation to fully realize the promise of bispecific antibodies in the global therapeutics market.
To know more about bispecific antibodies, visit @ Bispecific Antibody Production
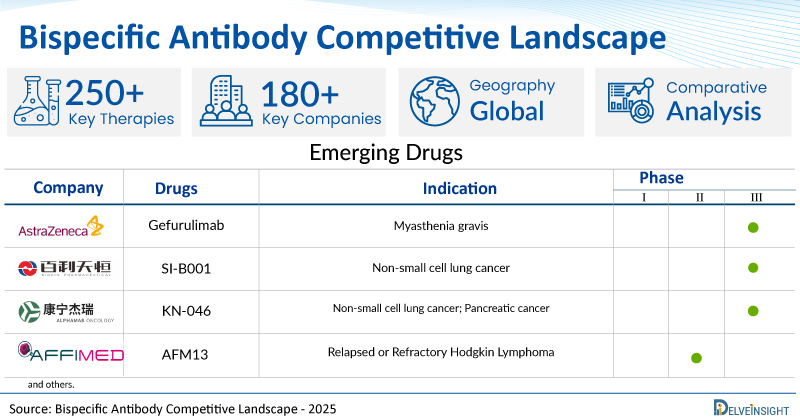
A snapshot of the pipeline bispecific antibodies mentioned in the report:
| Bispecific Antibodies | Company | Phase | Indication |
| Gefurulimab | AstraZeneca | III | Myasthenia gravis |
| SI-B001 | Sichuan Baili Pharmaceutical | III | Non-small cell lung cancer |
| KN-046 | Alphamab | III | Non-small cell lung cancer; Pancreatic cancer |
| AFM13 | Affimed GmbH | II | Relapsed or Refractory Hodgkin Lymphoma |
| IBI322 | Innovent Biologics | II | Hematological malignancies; Solid tumors |
| CTX-8371 | Compass Therapeutics | I | Non Small Cell Lung Cancer |
| MGD024 | MacroGenics | I | Hematological malignancies |
Discover more about bispecific antibody development @ Bispecific Antibody Clinical Trials
Key Developments in the Bispecific Antibody Domain
- In July 2025, Instil Bio announced the clearance of an Investigational New Drug (IND) application for AXN-2510, a PD-L1xVEGF Bispecific Antibody, for a Phase I Trial in Relapsed/Refractory Solid Tumors by the US Food and Drug Administration.
- In June 2025, Zai Lab Limited announced new data from its preclinical study of ZL-1503, the Company’s promising IL-13/IL-31R bispecific antibody, demonstrating its ability to simultaneously suppress the inflammatory and pruritogenic (itch-causing) pathways in atopic dermatitis (AD).
- In June 2025, BioNTech announced that the companies have agreed on the global co-development and co-commercialization of BioNTech’s investigational bispecific antibody BNT327 across numerous solid tumor types. Under the agreement, BioNTech and BMS will work jointly to broaden and accelerate the development of this clinical candidate.
- In May 2025, Minghui Pharmaceutical announced the successful dosing of the first patient in a Phase II clinical trial assessing the safety and effectiveness of an investigational combination therapy. The trial involves MHB039A, a PD-1xVEGF bispecific antibody, and MHB036C, a TROP-2-targeting antibody-drug conjugate, in patients with advanced non-small cell lung cancer (NSCLC).
- In May 2025, Pfizer Inc. announced an exclusive global licensing agreement, excluding China, with 3SBio, Inc. (01530.HK), a prominent Chinese biopharmaceutical company. The agreement covers the development, manufacturing, and commercialization of SSGJ-707, a bispecific antibody that targets PD-1 and VEGF. SSGJ-707 is currently in multiple clinical trials in China for non-small cell lung cancer, metastatic colorectal cancer, and gynecological tumors. Early data indicate promising efficacy and safety. 3SBio intends to launch the first Phase 3 trial in China in 2025.
- In April 2025, Roche announced that the European Commission had granted approval for COLUMVI (glofitamab) in combination with gemcitabine and oxaliplatin (GemOx) to treat adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), not otherwise specified, who are not eligible for autologous stem cell transplant (ASCT).
- In March 2025, Ocean Biomedical, Inc. announced that the China National Intellectual Property Administration (CNIPA) had issued a notice of patent grant for its bispecific antibodies targeting CHI3L1 and PD1, which are engineered to boost T cell-driven cytotoxic activity against tumor cells.
- In March 2025, Integral Molecular announced that the first patient had been dosed in a Phase 1 clinical trial of its out-licensed anti-Claudin 6 (CLDN6) bispecific antibody, CTIM-76. The trial is being conducted by its licensing partner, Context Therapeutics Inc., as part of a Phase 1 dose escalation and expansion study involving patients with advanced or metastatic ovarian, endometrial, and testicular cancers.
- In February 2025, Rondo Therapeutics announced that preclinical data on RNDO-564, a novel CD28 x Nectin-4 costimulatory bispecific antibody for advanced bladder cancer, would be featured in a poster presentation at the 2025 ASCO Genitourinary (GU) Cancers Symposium, held from February 13 to 15 in San Francisco, CA.
- In January 2025, Orion Corporation and Invenra Inc. announced a research collaboration to develop bispecific antibodies utilizing Invenra’s B-Body® platform.
- In January 2025, Biocytogen and Acepodia announced a landmark strategic collaboration to co-develop and evaluate a dual-payload bispecific antibody-drug conjugate (BsAD2C) program.
Scope of the Bispecific Antibody Competitive Landscape Report
- Coverage: Global
- Key Bispecific Antibody Companies: Instil Bio, Akeso Biopharma, Janssen, Amgen, Akeso, Zymeworks, Roche, IGM Biosciences, MacroGenics, Provention Bio, Jiangsu Alphamab Biopharmaceuticals, Sichuan Baili Pharmaceutical, Regeneron Pharmaceuticals, Boehringer Ingelheim, ABL Bio, Zymeworks, Compass Therapeutics, EpimAb Biotherapeutics, Betta Pharmaceuticals, Affimed Therapeutics, Novo Nordisk, and others
- Key Bispecific Antibodies in Pipeline: AXN-2510, AK-104, Amivantamab, Blinatumomab, Ivonescimab, Zanidatamab, Glofitamab, Imvotamab, MGD024, PRV 3279, KN-046, SI-B001, REGN-5458, BI-905711, ABL 102, ZW171, CTX-8371, EMB-01, MCLA-129, AFM13, Mim8, and others.
Table of Contents
| 1. | Bispecific Antibody Pipeline Report Introduction |
| 2. | Bispecific Antibody Pipeline Report Executive Summary |
| 3. | Bispecific Antibody Pipeline: Overview |
| 4. | Bispecific Antibody Marketed Drugs |
| 4.1. | Amivantamab: Johnson & Johnson Innovative Medicine |
| 5. | Bispecific Antibody Clinical Trial Therapeutics |
| 6. | Bispecific Antibody Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Bispecific Antibody Pipeline: Late-Stage Products (Phase III) |
| 7.1 | Gefurulimab: AstraZeneca |
| 8. | Bispecific Antibody Pipeline: Mid-Stage Products (Phase II) |
| 8.1. | Imvotamab: IGM Biosciences |
| 9. | Bispecific Antibody Pipeline: Early-Stage Products (Phase I) |
| 9.1. | MGD024: MacroGenics |
| 10. | Bispecific Antibody Pipeline: Preclinical and Discovery Stage Products |
| 11. | Bispecific Antibody Pipeline Therapeutics Assessment |
| 12. | Inactive Products in the Bispecific Antibody Pipeline |
| 13. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 14. | Unmet Needs |
| 15. | Bispecific Antibody Market Drivers and Barriers |
| 16. | Appendix |
For further information on bispecific antibody production, reach out @ Bispecific Antibody Applications
Related Reports
Bispecifics Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key bispecific antibody companies, including Provention Bio, Jiangsu Alphamab Biopharmaceuticals, Sichuan Baili Pharmaceutical, Regeneron Pharmaceuticals, among others.
Bispecifics/Trispecifics Market
Bispecifics/Trispecifics Market Forecast and Competitive Landscape – 2035 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key bispecifics/trispecifics companies, including Akeso, Summit Therapeutics, Zymeworks, BeiGene, Jazz Pharmaceuticals, Merus, Roche, among others.
CAR T-Cell Therapy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CAR T-cell therapy companies, including CytoAgents, Genentech, Incyte Corporation, Caribou Biosciences, Chimeric Therapeutics, Allogene Therapeutics, Kyverna Therapeutics, iCell Gene Therapeutics, Synthekine, Janssen Research & Development, SecuraBio, ImmPACT Bio, A2 Biotherapeutics, Gracell Biopharmaceuticals, Wugen, Sana Biotechnology, Oncternal Therapeutics, Vor Biopharma, CARsgen Therapeutics, Autolus Limited, Arcellx, Kite, Novartis, Lyell Immunopharma, ImmPACT Bio, Tmunity Therapeutics, Poseida Therapeutics, Precigen, AffyImmune Therapeutics, 2seventy bio, Takeda, Cartesian Therapeutics, Cabaletta Bio, Legend Biotech, Miltenyi Biomedicine, Beam Therapeutics, among others.
CAR T-Cell Therapy Competitive Landscape
CAR T-cell Therapy Competitive Landscape – 2025 report provides comprehensive insights about the pipeline and current landscape, pipeline and marketed drug profiles, and the key CAR T-cell therapy companies, including Alnylam Pharmaceuticals, JW Therapeutics, Gilead Sciences, Janssen Pharmaceuticals, Innovent Biologics, Sorrento Therapeutics, Cartesian Therapeutics, CASI Pharmaceuticals, Juventas Cell Therapy, Novartis, Poseida Therapeutics,Shanghai Unicar-Therapy Bio-medicine Technology, Sinobioway Cell Therapy, Tessa Therapeutics, Wuhan Bio-Raid Biotechnology, Miltenyi Biomedicine, Bristol-Myers Squibb, Autolus Limited, Beijing Immunochina Medical Science and Technology, Carsgen Therapeutics, Cellular Biomedicine Group, Chongqing Precision Biotech, Eureka Therapeutics, Formula Pharmaceuticals, Guangzhou Bio-gene Technology, Hebei Senlang Biotechnology, Mustang Bio, MolMed, Aurora BioPharma, Atara Biotherapeutics, Autolus, Bellicum Pharmaceuticals, Kecellitics Biotech Company, Yake Biotechnology, Minerva Biotechnologies, Allogene Therapeutics, PersonGen BioTherapeutics (Suzhou), Precision BioSciences, Pregene (ShenZhen) Biotechnology Company, Shanghai GeneChem, Shanghai Longyao Biotechnology, Shenzhen BinDeBio, among others.
Monoclonal Antibodies Competitive Landscape
Monoclonal Antibodies Competitive Landscape – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key monoclonal antibody companies, including Novartis, Gmax Biopharm, Omeros Corporation, Merck Sharp & Dohme, Disc Medicine, Eledon Pharmaceuticals, Alexion AstraZeneca Rare Disease, Chinook Therapeutics, Omeros Corporation, among others.
Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.

Contact Us Shruti Thakur info@delveinsight.com +14699457679
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
